Corneal Cross-Linking
Treatment for Progressive Keratoconus
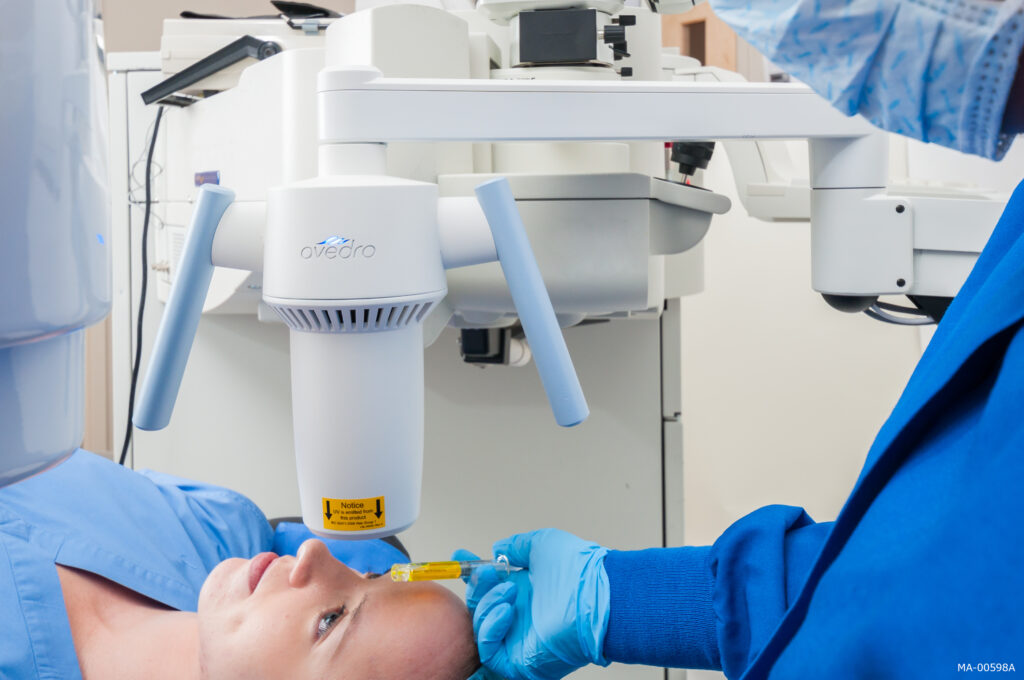
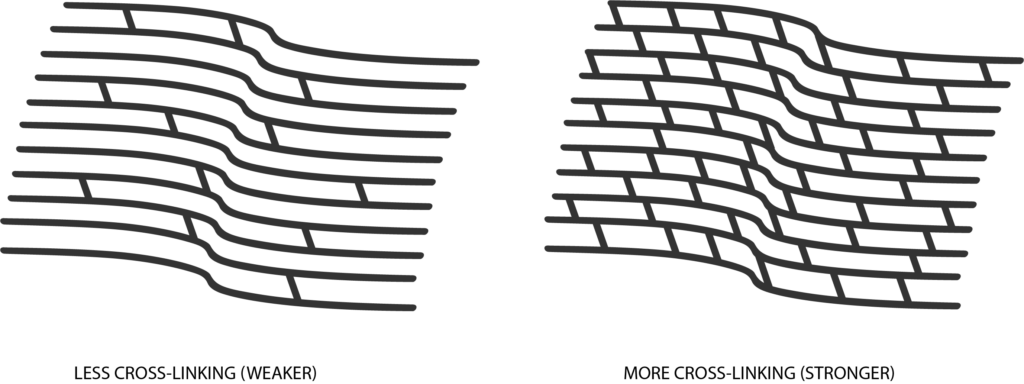
Corneal cross-linking is a minimally invasive procedure to treat progressive keratoconus, a condition in which the cornea thins and bulges into a cone-like shape, causing vision distortion. iLink FDA approved epi-off cross-linking combines the use of prescription eye drops, Photrexa® Viscous, Photrexa®, and ultra-violet A (UVA) light from the KXL® system. This combination promotes the creation of new collagen bonds, reinforcing the cornea’s structure and preventing further deterioration.
What to Expect with Corneal Cross-Linking
The goal of the procedure is to slow or prevent further progression of the condition and preserve vision.
FAQ
Take the First Step: Find Out if iLink May Be Right for You
If you’re ready to take control of your vision and slow or halt the progression of keratoconus, iLink corneal cross-linking could be the answer. Call us today to request your a consultation. Dr. Kenneth Himmel, our Corneal expert who specialize in this procedure. He will perform a comprehensive eye exam including advanced corneal imaging and topography in order to determine if you are a candidate for the iLink cross-linking procedure.